Conformer-specific polar Diels–Alder cycloadditions published and selected as Highlight by Nat. Comm.
18 October, 2021
There has been a long-standing discussion whether Diels–Alder cycloadditions proceed via stepwise or concerted mechanisms. By combining a conformationally controlled molecular beam with trapped ions, we explored the mechanism of the model polar cycloaddition of conformer-specific 2,3-dibromo-1,3-butadiene with propene ions under single-collision conditions in the gas phase. Both conformers of the diene are reactive with capture-limited reaction rates and a simultaneous competition of concerted and stepwise reaction pathways was found.
Our results from collaborative experiments between our CFEL-CMI and the Willitsch-group at Universität Basel were supported by theory from the von Lilienfeld-group in Basel and published in the article Conformer-specific polar cycloaddition of dibromobutadiene with trapped propene ions. These important results results are featured as Editors' Highlight by Nature Communications.
The Diels–Alder (DA) cycloaddition is one of the key reactions in synthetic organic chemistry for generating cyclic products. Here, a conjugated diene and an alkene, the dienophile, react to form a cyclohexene compound. It was first described by Otto Diels and Kurt Alder in the 1920s, who were later awarded the Nobel Prize in Chemistry in 1950. Since its discovery, much work contribute to understanding its mechanism in detail. However, there has been a long-standing discussion whether these reactions proceed via stepwise or concerted mechanisms.
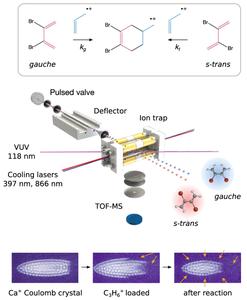
The two research groups headed by Professor Stefan Willitsch from the Universität Basel and by Professor Jochen Küpper from the DESY and the Universität Hamburg have investigated the conformation specificities of the Diels-Alder reaction, i.e., their distinct abilities to undergo the chemical reaction and at which rate. The model polar cycloaddition of 2,3-dibromo-1,3-butadiene (DBB) with propene ions was chosen to explore the Diels-Alder reaction mechanism under single-collision conditions in the gas phase. The separation of two conformers of the diene was achieved using the electric deflector, an “electric prism” for molecular quantrum states developed by Professor Jochen Küpper's group at CFEL, DESY and Universität Hamburg. The scientists delivered a molecular beam containing two conformers of DBB through an inhomogeneous strong electric field. The gauche- and s-trans-DBB were deflected differently due to their distinct dipole moments, allowing to separate them in space and obtain gauche and s-trans samples. Using this approach, the researchers were able to perform conformer-controlled reactions between the specific conformers of the diene and ultracold trapped propene ions. During this process a six-membered ring is formed.
In the current work we demonstrated that both conformers of the diene, gauche and s-trans, were reactive with capture-limited reaction rates. In collaboration with the group of Professor Anatole von Lilienfeld, Universität Basel and Universität Wien, quantum-chemical simulations of the system were performed, which suggest that both limiting scenarios – concerted and sequential – of the DA reaction mechanism are simultaneously active in the present system. We rationalized this by a simultaneous competition of concerted and stepwise reaction pathways, revealing an interesting mechanistic borderline case.
Our work demonstrates a platform for probing conformer-specific chemical kinetics in model systems of fundamental organic name reactions under controlled conditions in the gas phase. This capability opens up opportunities for the precise investigation and validation of reaction mechanisms.